Project To Develop A New Mercury Chelator:
Goal:
Motivate the development of a new mercury chelator (because current offerings do more harm than good), for the purpose of mercury detoxification. With current mercury chelator options falling short, there is a compelling opportunity for pioneering chemists and research teams to lead an effort to develop a safer and more effective mercury detoxification process. Help shape a healthier world while unlocking the immense potential for societal impact by championing this mission.
Patients:
All humans are exposed to some level of mercury intoxication. The environment of planet Earth has become irreversibly polluted with the neurotoxin, mercury. The burning of coal releases ethyl-mercury into the atmosphere which distributes it worldwide. Mercury has entered the ecosystem of the oceans, poisoning fish which gets consumed by humans (EPA advisories). Products such as Compact Fluorescent Light Bulbs (CFLs) when broken release mercury as do thermometers and thermostats which may employ mercury. The extraction of gold in "artisanal" small scale mines, use and release an estimated 2000 tons of mercury into the environment annually (ref UN). The widespread use of silver-mercury amalgam dental fillings (estimated 91,000,000 in US in 2018 survey) has put a mercury leaching time-bomb into the mouths of many.
National Health Statistics data estimates that more than 100,000 Americans receive chelation each year and far fewer than 1 % of these cases are managed by medical toxicologists (ref). This statistic emphasizes the need for safe and effective chelation therapies.
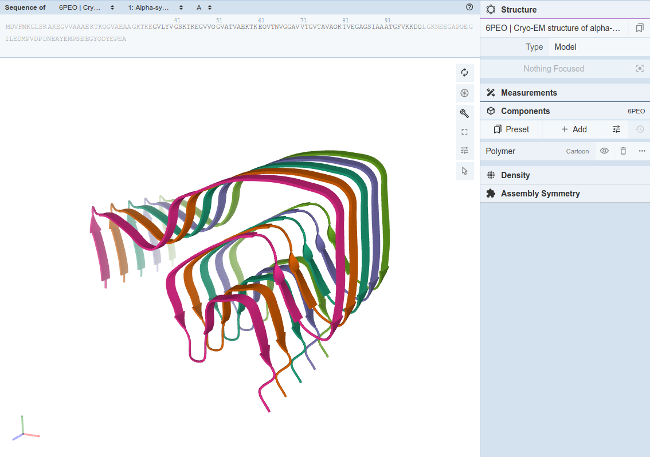
Some recently published papers address the link between mercury intoxication and alpha-synuclein fibrillation and what is known as "Idiopathic Parkinson's disease" (references: Mercury and Parkinson's Disease: Promising Leads, but Research Is Needed (Torrey and Simmons, 2023), The toxic metal hypothesis for neurological disorders (Pamphlett and Bishop, 2023)). Mercury intoxication can also cause tremors, movement disorders, loss of balance, loss of coordination, rigidity, etc which falls under the category of "Parkinsonism" where the patient displays the behavior of Parkinson's disease but may not have the pathology of Lewy bodies or alpha-synuclein aggregation.
Murcury is also implicated with cognitive disorders, anxieties, headaches, infertility, autoimmune dysfunctions, hypertension, atherosclerosis, stroke, coronary heart disease, myocardial infarction, renal dysfunction, renal insufficiency, and proteinuria (ref).
Unfortunately the natural human detoxification pathways do not process mercury well, hence the need for a chelator which can bind with mercury and provide the chemical properties to allow the resulting compound to be processed and excreted.

Hg is derived from the Latin name for mercury: hydrargyrum
Problem:
Mercury Chelation Currently Does Not Work:
Current chelator compounds are either ineffective at removing mercury from the body or they are ineffective at maintaining a bond with mercury resulting in the release of mercury. When a chelator drops mercury before being excreted, it allows mercury to relocate to sensitive areas of the body such as the brain. A study by H. V. Aposhtian et al., found that current chelators (DMPS, DMSA, glutathione (GSH) and lipoic acid), alone or in combination, do not decrease the brain or organ (liver, kidney, ...) burden of mercury.
References:- The following paper tests DMPS, DMSA, GSH, vitamin C, and lipoic acid, alone and in combination, and concludes that they do not decrease brain mercury in rats exposed to elemental mercury vapor:
Vitamin C, Glutathione, or Lipoic Acid Did Not Decrease Brain or Kidney Mercury in Rats Exposed to Mercury Vapor (H. Vasken Aposhian et al., 2003)
Mercury Release and Relocation:
A study by the researcher, Graham George, showed that current chelators such as DMPS or DMSA do not maintain a bond with mercury and do not bond in a fashion expected by chemists. The weak binding of DMPS and DMSA is due to the fact there is not enough stereo-chemical space between the two thiols located on adjacent carbons to allow for binding a mercury atom. Pairing one mercury atom with one DMPS or DMSA molecule doesn't fit. Thus DMPS and DMSA stir up mercury, where it then travels to high mercury affinity areas like the brain. Many of the chelators in use today were developed in the 1940s and 1950s and are ineffective or outright dangerous due to mercury release and relocation.
Mercury chelation does not work!
References:- Mercury binding to the chelation therapy agents DMSA and DMPS and the rational design of custom chelators for mercury [PDF] (Graham George et al 2004)
Mercury Excretion:
Once the chelator has bonded with mercury, it has to be recognized by the kidneys or liver so that it may be excreted from the body as urine or as stool respectively. Not all chelators are effective at excreting mercury. This is true for the chelator NBMI which has been shown to fail at this task. Some presume that the cytochrome P450 enzymes break down NBMI before being excreted, permitting mercury to remain.
References:- Amelioration of Acute Mercury Toxicity by a Novel, Non-Toxic Lipid Soluble Chelator NBMI: Effect on Animal Survival, Health, Mercury Excretion and Organ Accumulation (Haley et al, 2012)
"The most significant finding was that NBMI treatment did not reduce the mercury levels in the organs of rats treated with NBMI."
"NBMI treatment administered to rats acutely exposed to toxic levels of Hg did not significantly alter Hg excretion in urine or feces"
Pharmacokinetics:
Pharmacokinetics is the study of how drugs and foreign compounds are processed by the body. While the liver is the primary purveyor of enzyme production used to detoxify the body, the kidneys and most organs also possess some ability to break down foreign matter. The liver excretion pathway has multiple phases, the first of which is to chemically alter the foreign compound to make it less toxic and manageable (Functionalization), followed by a chemical reaction to make it more water soluble so that it can be excreted (Conjugation). Industrial compounds such as NBMI are excellent for bonding and removing mercury from water but are not useful in the human body as a chelator as the body produces enzymes that may break down the chelator before it has a chance to remove it from the body.
There are many enzymes at work, but none have been studied to the level required to understand what actually happens to the chelator or how to make it resilliant enough to excrete mercury but still able to be broken down so that it does not build up to toxic levels.
Partial list of enzymes produced by the body to break down foreign compounds:- Phase I: Functionalization / Biotransformation
- UGT: Uridine Diphospho Glucuronyl Transferase
- AD: Alcohol Dehydrogenase
- ADs: Aldehyde Oxidases
- FMO: Flavin Mono Oxidases
- ALDHs: Aldehyde Dehydrogenases
- MAO: Mono Amine Oxidases
- XOs: Xanthine Oxidases
- NRs: Nitro Reductases
- AR: Azo Reductase
- Esterase(s)
- Amidase(s)
- Cytochrome P450: (responsible for 90% of foreign metabolism)
- CYP3A4: The most common enzyme, involved in metabolizing over 50% of all drugs and foreign compounds.
- CYP3A5
- CYP2D6
- CYP2C9
- CYP2C19
- CYP1A2:
P450 pathways also respond to inhibitors and inducers. Inducers are sometimes used to decrease a drug's effectiveness by increasing the metabolic activity of liver enzymes. Inhibitors are sometimes used to increase a drug's effectiveness by keeping them in the body longer:
eg. grapefruit juice, clarithromycin, ketoconazole, ritonavir, and fluoxetine.
- Phase II: Conjugation / Neutralization
- ST: Sulpho Transferase
- MT: Methyl Transferase
- COMTs: Catechol-O-Methyl Tansferase
- NATs: N-Acyl Transferases
- ATs: Acyl Transferases
- PS: Phospho Transferase
- GST: Glutathione Sulpho Transferase
It is also important that the chelator possess water-soluble traits so that it can enter the body and navigate tissues to find and bind with mercury and eventually exit the body, carrying the mercury out. This is often accomplished by adding polar molecular traits to the chelator compound.
The Brain Attracts Mercury:
The substance responsible for pigmentation of the skin is known to be melanin. More melanin results in darker skin. Highly pigmented neurons of the brain, like the substantia nigra, are also given their color from the presence of melanin, more specifically, neuromelanin. Studies have shown that elemental analyses of neuromelanin revealed a high sulfur content. Sulfur in turn has a high affinity for mercury. It is this sulfur affinity that most chelators rely upon to bond with mercury. This affinity also draws mercury to the neurons of the substantia nigra, a part of the brain responsible for movement. A chelator should form a bond with mercury that is stronger than that of neuromelanin. Reference:- Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour (2001)
"... neuromelanin is indeed a genuine melanin because it has a stable free radical structure and avidly chelates metals."
"Elemental analyses of neuromelanin revealed a high sulphur content ..."
"Neuromelanin from the substantia nigra can interact with many heavy metal ions such as zinc, copper, manganese, chromium, cobalt, mercury, lead, and cadmium; in addition, it binds iron particularly strongly."
For more discussion on mercury, chelation and Parkinson's see Mercury and Parkinson's Disease.
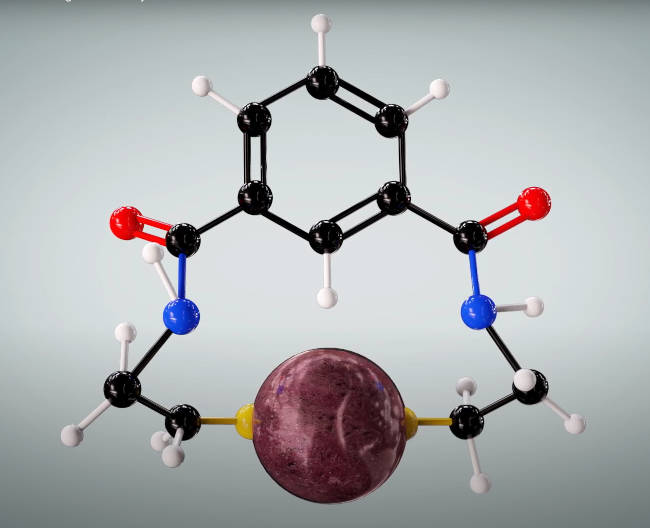
NBMI: this chelator bonds to the mercury atom (180 deg) but fails to be excreted from the human body
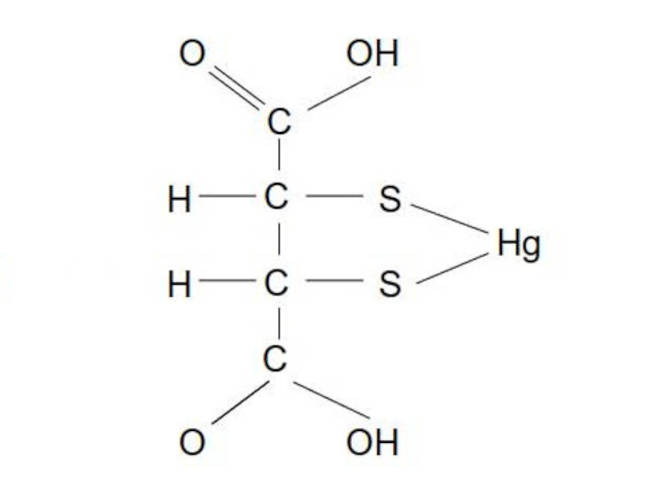
DMSA: this chelator does not typically bond in this geometric structure due to mercury's propensity for a 180 deg bond.
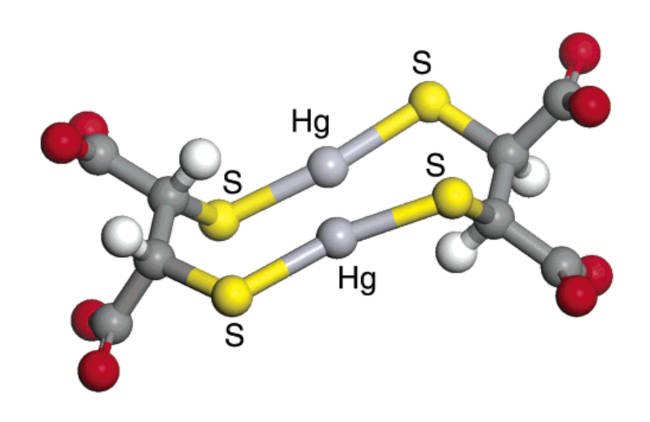
DMSA: Research by Graham George shows that the typical DMSA chelate structure with Hg is 2 DMSA bonding with 2 Hg. This chelator gets excreted from the body, primarily by the kidneys, but makes a structurally weak bond to the mercury atom and generally drops it before being excreted
Requirements For A New Chelator:
- Must be soluble in water or fat (lipid) to be readily absorbed and transported in the body's fluid or tissues.
- Must be able to administer by IV, injection or orally.
- Must have a high affinity for mercury: must be able to break the bond mercury has with body tissue to prefer making a bond with the chelator.
- Must be non-toxic, not carcinogenic, not harmful in both conditions (1) when bound to mercury and (2) when unbound.
- Must not compete with binding at sites that are meant for natural compounds to prevent any negative biological activity
- Must be able to form a chelate (the resulting compound of the chelator bonded with mercury) which the body can excrete via the kidneys or via the liver
- Must be resistant to biotransformation from the metabolic processes of the body so that it does not lose its effectiveness or become toxic or generate toxic byproducts.
- Must be able to effectively bind mercury across the range of pH levels found in the body,
- Once bonded with mercury, the chelator must not drop this bond to release the mercury, allowing redistribution. The release of Mercury often occurs if the bond is geometrically awkward or if the chelation compound is disolved by enzymes such as cytochrome P450, before being excreted, leaving mercury to remain.
- Once bonded with mercury, the chelator-mercury complex must able to be efficiently expelled via the kidneys or liver and not aggregate in the body.
- Must be able to maintain a half-life long enough to be able to bind and extrete mercury but not so long that it accumulates to undesirable levels.
- Must be able to pass through cell walls or draw mercury through cell walls to bond with and remove mercury wherever it is
- There is no requirement that a working chelation treatment be solved by a single compound. A combination of hydrophilic and lipophilic compounds or small and large molecules which may or may not pass through the B-B-B are all valid if the resulting treatment works.
- Must be able to pass through the blood-brain-barrier (BBB)
- Must be able to have an affinity to mercury higher than that of brain tissue. Note that in contrast to light grey brain matter, dark brain matter has higher levels of neuromelanin which is high in sulfur. Sulfur is typically used in traditional chelators due to its affinity for mercury. May need multiple sulfur bonds or may need to employ selenium as the atom for bonding with mercury
- Must be able to pass through the neuron cell walls or draw mercury through neuron cell walls to bond with and remove mercury
- A chelator which can make two points of attahment (bidentate) or more (multidentate) is preferred as it will be a stronger bond with the mercury atom than a single point of attachment.

Chelator Validation:
A chelator requires two tests to validate its performance:- does it remove mercury
- does it lower the body burden of mercury without redistributing it to the brain or other sensitive organs
Mercury Formulations:
Mercury can take each of the following forms:- elemental mercury (Hg)
- organic mercury (e.g. MeHg)
- inorganic mercury (e.g. HgCl2)
A chelator should be tested for efficacy for all forms of mercury or at the very least, the limitations of the chelator should be known.
Redistribution:
A test to see if the chelator is not losing its bond and redistributing mercury to the brain, is also required and can only be performed on mice as an autopsy. An autopsy is required to measure the brain burden of mercury for both the mice testing the chelator and for the control specimens. This test is required to determine if the chelator is lowering or increasing the brain burden of mercury or is ineffective.
The following paper is a good example of chelator validation using mice: Vitamin C, Glutathione, or Lipoic Acid Did Not Decrease Brain or Kidney Mercury in Rats Exposed to Mercury Vapor (Aposhian et al., 2003)
This paper covers the mercury intoxication of mice, the measurement of the effects of chelation on the mouse brain and the effects of mercury mobilization.
The paper also covers compliance with animal treatment guidelines as well as the use of control and sentinal specimens.
Challenge and Burden Tests:
A urine test or stool test can be used as part of a challenge test where measurements can be made before and after use of the chelator. This test is used to verify whether the chelator is excreting mercury via urine or stool. If the chelator is excreting mercury, the mercury measured in urine or stool should be much higher after a dose of chelator is administered.
Many believe that a blood test, urine test, stool test or hair test can stand alone as valid tests of mercury burden but they can not. Mercury typically binds to tissue and does not move freely enough to be adequately measured in a blood test. In addition to the lack of freely available mercury, the biological processes associated with urination, passing stool or growing hair do not possess the ability to quantify the level of mercury burden. Thus these tests are only useful as part of a "challenge test" (also known as a "provocation test") to see if a chelator is removing mercury and not as a stand-alone test to measure the level of mercury intoxication.
Unfortunately there are no challenge test standards to determine the level of mercury intoxication based on a body mass normalized dosage of a chelator nor is there any consideration given to the sensitivity of affected organs.
The development of a new working chelator is most probably a much simpler task than trying to find a way to measure one's mercury burden, especially one's brain burden. We fear that the development of a new and effective chelator will not make any progress if measurement is prioritized over removal.
Investigation:
There are some chelators which have not yet been shown to be validated as safe from redistributing mercury. There are also some which have been improperly validated. No chelator has been found to lower the brain burden of mercury.
Chelators not tested or not adequately tested:- NBMI: C12H16N2O2S2
- miaDMSA
- Chlorella (identify the compounds responsible for bonding with mercury)
- Selenium supplements (study should include research into the pharmacokinetics of mercury selenide HgSe, its formation and eviction, if it occurs)
- Cysteamine: C2H7NS
- Acetylcysteine: (ref)
- Garlic: Active ingredients: Allicin (C6H10OS2), Diallyl sulfide (DAS: C6H10S) (one sulfur atom), Diallyl disulfide (DADS: C6H10S2) (two sulfur atoms)
- Chelators proposed by G. George et. el (Towards a custom chelator for mercury):
- A alkane dithiolate chelator (fig 4): S(CH2)nS where n=4, 6, 8 or 10
- Two-coordinate mercuric ions coordinated by thiolate ligands (fig 1): (CH3S)2Hg
- Three-coordinate mercuric ions coordinated by thiolate ligands (fig 6): (CH3S)3Hg
- Polymeric: S(CH2)nS–Hg–S(CH2)nS–Hg
- Three coordinate case: Hg(SR)3
- HgMetX: benzene-1,3-diamidoethanethiolatomercury(II) (fig 5)
(also with ring substitutions carboxylate or sulfonate) - Trithiopod: benzene-1,3,5-triamidopropanethiolate (fig 9)
Chelator Development Using Computational Chemistry Tools:
Many of the chelation compounds in use today were developed during the WW2 era or at the very least, prior to the availability of tools such as "Computational Chemistry". These tools allow for the simulation of chemical reactions, compound optimization, compound discovery, virtual screening (VS) and high throughput screening (HTS). They are ideally suited for the discovery, simulation and optimization of potential chelators including the optimization and rework of current chelators. One key Computer-Aided Drug Design (CADD) feature is "docking" or the modeling of binding energy to a target where the bond of the chelator to mercury can be simulated. The virtual world allows for rapid development and experimentation.
With the current generation of software tools one can simulate the pharmacokinetics and model the clearance mechanisms using ECCS (Extended Clearance Classification System), model the Blood-Brain-Barrier (BBB) permeability, predict solubility and test for drug-drug interactions (transporter inhibition where transporter-in models the stomach and transporter-out include kidneys and the liver). One could begin with an existing chelator and optimize it using CADD to improve one of its flaws such as clearance properties. One could also virtually substitute Selenium for Sulfur to attempt to increase its bond to mercury.
The newer iterations of drug design software are including machine learning infrastructure to automate the optimization of pharmaceutical compounds. These design, optimization and simulation tools offer researchers a significant development advantage and should not be overlooked.
Computer-Aided Drug Design (CADD) software:- Schrodinger: Maestro - computational chemistry, molecular design, drug formulation and visualization
Often teams with pharma drug development programs. - Wavefun: Spartan - computational chemistry and visualization
- Biovia (Dassault Systemes):
- Biovia Discovery Studio - biological and physicochemical 3D modeling and simulation
- Discovery Studio Visualizer - free download for MS/Win and Linux
- Biovia Materials Studio - Chemical and Catalyst R&D
- Biovia Discovery Studio - biological and physicochemical 3D modeling and simulation
- Cressset
- Pharmacelera
- OpenEye Scientific
- Chemical Computer Group
- Swiss-Model - 3D protein modeling
- Acellera
Compounds developed can be shared digitally in any of the following public databases:
- PubChem - chemical information
One can also download current chelator compound digital models (DMSA, DMPS, NBMI, ALA, MiaDMSA, Glutathione, etc) - BindingDB - molecules with drug-like properties and their binding affinities
Chelator Creation:
While the virtual world of computational chemistry allows for rapid prototyping, at some point the chelator compound must be created in order to be tested or applied. There are many university and commercial labs that can perform this task.
- PCI
- Abbvie Contract Manufacturing
- Fandachem (China)
- McGuff
- Alta Sciences CDMO
(Contract Development and Manufacturing Organization) - Curia

Clinical Trials:
A new chelator could be used under "compassionate use" also known as "expanded access programs" (EAPs), "right to try", as a supplement which makes no claims, or get FDA phase 1, 2 and 3 approval.
FDA Compassionate Use:
This protocol relates to the use of an investigational medicine outside of a clinical trial to treat a serious or life-threatening condition. Mercury toxicity is often such a condition. A request for "Compassionate Use" or "Expanded Access" must be requested by a licensed doctor and approved by the FDA for an individual. Requests are made by phone or email. Adverse events must be immediately reported to the FDA.
EAPs conditions of use:- has a serious and life-threatening condition
- has no other comparable treatment option
- has exhausted approved treatment options
- likely to have benefits that outweigh the risks
- if drug is in clinical trials but the patient does not qualify
See FDA expanded access contact information and forms.
Right To Try:
A US law passed in 2018 provides another way to access unapproved drugs. Right to try is only for drugs in a FDA phase 1 trial. This law is used to request access to an unapproved drug from a company that makes it, without having to go through the FDA.
FDA Phase 1, 2, 3 Trials:
Consultants and contractors:
- ProPharma
- Consano - clinical research trials
- OPKO Health - medical test and diagnostics
- FDA Compliance Group
Potential Sources of Funding:
We have a chicken and egg problem. Those financing an endeavor want to see a research team who will develop the chelator. It's hard to put together a scientific team when we can't identify financing.
Potential Funding Sources:- NIH: National Institute of Health - (USA)
- US Defense Department - (USA)
- MRC: Medical Research Council - (UK)
- European Commission: Research and Innovation - (EU)
- NHMRC: National Health & Medical Research Council - (AU)
- Welcome Trust - (UK)
- CIHR: Canadian Institutes of Health Research - (Canada)
- German Research Foundation - (DE)
- U.S. Department of Veterans Affairs - (USA)
- NSF: National Science Foundation - (USA)
- WHO: World Health Organization
- United Nations:
- Crowd Funding:
Medical Research Crowd Funding:
- Experiment - fund research
- Curie.bio - funds startup companies
List of research funding sources: Health Research Funding
Is crowdfunding a viable source of clinical trial research funding?

Call To Action:
This web page is meant to instigate and inspire a call to action to take a journey towards pioneering the development of a new mercury chelator. The demand is urgent. It is hoped that development teams of chemists and biochemists can be formed to harness the power of modern computational chemistry tools to engineer a breakthrough solution for a safe and effective mercury chelation compound. This call to action is dependent upon the expertise and passion of dedicated scientists and research teams to bring this vision to life. University based research teams are, now more than ever before, in dire need of funding for research projects. Now may be an opportune time to put together and employ a team of students and professors. Join us to unite and rally passionate scientists and experts to collaborate on the quest to safeguard global health and environmental well-being from the harmful effects of mercury.
Useful skills:
- chemistry / biochemistry for basic compound structure
- pharmacokinetics: drug absorption, distribution, metabolisim and elimination
- computational chemistry: optimize bond gemetries, bond lengths, density functional theory to minimize structure energy
- cytochrome P450: enzyme reactions that may affect a chelator's duration of effectiveness. A chelator must last long enough to evict mercury before being neutralized.
- spectroscopy: chemical analysis to confirm the formation, geometry and stability of the chelator, chelator-mercury chelate complex and the presence of molecules generated as they are broken down: Gas Chromatography-Mass Spectrometry (GC-MS), X-ray Absorption Spectroscopy (XAS/XANES/EXAFS).
- toxicology: Evaluating the safety and potential side effects of the chelator to ensure that it doesn't cause more harm than good.
Links:
Discussions on the development of new chelators.- Towards a custom chelator for mercury: evaluation of coordination environments by molecular modeling (George et al, 2011)
A technical discussion on the use of molecular dynamics and computer simulations for the design of a new chelator. Includes design suggestions. - Clawing Back: Broadening the Notion of Metal Chelators in Medicine (K. Franz, 2012)
Status:
Not funded. Development team not identified.
Note that this website does not have the financial or technical means to undertake this call to action.

A listing on this web page does not mean that this website endorses any given business or institution.