Intermittent Fasting, Autophagy and Parkinson's Disease:
The theory behind "Intermittent Fasting" (also known as "Time Restricted Eating") is that if you reduce your food intake to fewer hours and increase the duration of fasting, you will force cells in your body into a mode known as "autophagy" during the fasting phase. It is while the cells are in a state of autophagy that the cells of the brain and central nervous systen will begin cleaning-up neuron killing alpha-synuclein protein aggregations, the mechanism of the neurodegeneration of Parkinson's disease.
The period of eating and digestion is called the "absorptive" or fed state. The tail end of the fasting portion of your day, called the "post-absorptive" state, will put your brain and nervous system in a state of metabolic stress where the body's internal cellular mechanisms will recycle the waste proteins responsible for Parkinson's Disease (alpha-synuclein Lewy Bodies) and Alzheimer's (Beta-amyloid protein and plaque). The result of the recycling of proteins results in the generation of amino acids that are reused by cells in the synthesis of new proteins. The theory maintains that during the eating phase, the cells of the body feed directly off of the glucose and carbohydrate nutrients consumed and available in your blood supply and the glycogen stores in the liver. This is called the metabolic state of glycolysis. In this state the blood glucose is elevated, inducing the body to produce more insulin which promotes the conversion of excess glucose into stored body fat.
During the fasting period the body runs out of the easily available fuel and switches to other sources. The body will also produce growth hormone which will promote lean muscle mass, stimulate the production of neurotrophic factors (BDNF: Brain Derived Neurotrophic Factors and FGF: Fibroblast Neurotrophic Factors) which promote neuron health (growth of axons, dendrites and increase in mitochondria) and the production of new neurons from stem cells (neurogenesis), improves one's cholesterol profile, lower blood pressure and lower insulin levels improving clairity of thought. During this fasting phase, nutrients are not available in the blood supply so the body enters a mode where it consumes excess fat found in the body and turns it into ketone bodies to fuel your body. This is a state known as ketosis where fat reserves of the body are released and consumed. Most cells in your body use ketones and glucose for fuel. For cells that can only process glucose, like parts of the brain, the glycerol derived from dietary fats is made into glucose by the liver in a process known as gluconeogenesis. During the fasting state, the body also enters a clean-up phase where the internal cellular mechanism for recycling, consumes plaque waste products. This catabolic break down and clean-up phase is referred to as Autophagy.
Autophagy:
Autophagy is a degradation process involved in the clearance of unnecessary or dysfunctional proteins and cellular components. Autophagy (specifically macroautophagy) begins with cellular stress, such as limited nutrients, which influences the the cell's lysosomes (internal cell component responsible for recycling cellular proteins, carbohydrates and lipids) to come to action and fuse with autophagosomes (generated when a phagophore engulfs the protein material to be processed) to form autolysosomes containing everything required to degrade, recycle and process the material. Autophagy is an adaptive cell survival process of consuming internal cell material during a period of nutrient deficiency. It is important that during the fasting phase that nutrition be held back from the blood supply otherwise the body will never reach Autophagy. It also must be pointed out that autophagy is the goal and that restricting a diet so much so that it becomes chronic starvation, can damage rather than protect neurons.
Research has also shown that the misfolded protein responsible for the generation of Lewy Bodies, alpha-synuclein, spreads via a cascade from neuron to neighboring neuron and that Intermittent Fasting mediates microglia (immune system macrophage cells in the brain which act as immune sentinels orchestrating an inflammatory response) to consume the alpha-synuclein. This has been termed "synucleinphagy".
Note that "intermittent fasting" and "calorie restriction" have become two ideological camps in the study of weight loss and dieting. The goal for one with Parkinson's disease (PD) is to reach a state autophagy in order to promote alpha-synuclein protein recycling and cell clean-up to prevent their accumulation and aggregation. As an added bonus, autophagy will also induce the clean-up of tau proteins responsible for the pathology of Alzheimer's disease. This is achieved with intermittent fasting. A calorie restricted diet may never reach a state of autophagy or ketosis for long eating cycles and may lead to muscle loss and a lack of essential nutrients. Intermittent fasting allows for weight or muscle gain or loss depending on the quantity of calories consumed, while still supporting the state of autophagy.
| BHB Millimolar | Description |
|---|---|
| < 0.2 | Body not in a state of ketosis. |
| 0.2 - 0.5 | Body in a state of mild ketosis. |
| 0.5 - 3.0 | Body in a state of induced nutritional ketosis. |
| 2.5 - 3.5 | Body in a state of post exercise ketosis. |
| 3.0 - 6.0 | Body in a state of prolonged fasting ketosis. |
| 15 - 25 | Body in a state of ketoacidosis. Note that this adversly affects the blood PH levels and requires medical attention. This is typically a result of medications, diabetes mismanagement, toxicity or poisoning and not fasting. |
See references:
- Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease (2017)
"As autophagy is one of the main systems involved in the proteolytic degradation of α-syn, pharmacological enhancement of autophagy may be an attractive strategy to combat α-syn aggregation in PD"
"Maintaining the balance between protective and detrimental effects is therefore of vital importance in therapeutic approaches to stimulate broad autophagy" - Autophagy – a cure for many present-day diseases? (2017)
- Role of Autophagy in Parkinson’s Disease (Cereri and Blandini, 2019)
DOI: 10.2174/0929867325666180226094351 - The Role of Autophagy in Parkinson’s Disease (2012)
DOI: 10.1101/cshperspect.a009357 - Effects of Intermittent Fasting on Health, Aging, and Disease: 6 hr eating/18 hr fast (2019)
- The Role of Intermittent Fasting in Parkinson's Disease Mayo Clinic, Front Neurol (Neth et al, 2021)
- Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors (2020)
"Fasting in combination with calorie restriction modulates molecular mechanisms such as m-TOR, FOXO, NRF2, AMPK, and sirtuins, ultimately leads to significantly reduced inflammatory marker levels, as well as improved metabolic markers." - Autophagy Journal
- Short-term fasting induces profound neuronal autophagy - data demonstrated that food restriction caused a profound upregulation of autophagy in the brain as confirmed by the abundance of autophagosomes in neurons of food-restricted mice. (2010)
DOI: 10.4161/auto.6.6.12376 - Synucleinphagy: a microglial "community cleanup program" for neuroprotection (2020)
DOI: 10.1080/15548627.2020.1774149
- Short-term fasting induces profound neuronal autophagy - data demonstrated that food restriction caused a profound upregulation of autophagy in the brain as confirmed by the abundance of autophagosomes in neurons of food-restricted mice. (2010)

Stages of Fasting:
| Hours | Description |
|---|---|
| 4 - 8 | Food is digested and has left the stomach. Blood sugar levels fall. |
| 12+ | Body enters state of Keytosis, burns body fat and lowers insulin and inflammation levels. Brain Derived Neurotrophic Factors (BDNF) generated.
References: |
| 18+ | Significant fat burning and keytone generation. Signaling to reduce inflammation and cell DMA damage repair.
References:
|
| 24+ | Fasting activates the AMPK signaling pathway and inhibits mTOR activity, which in turn activates autophagy.
Autophagy, cell recycling of misfolded alpha-synuclein and neuroprotective action.
Insulin levels drop.
References:
|
| 48+ | Autophagy becomes more abundant.
Growth hormone secretion becomes greater.
References:
|
| 54+ | Insulin drops to lower levels. Body becomes insulin sensitive. Lowers inflammation levels.
References: |
| 72+ | Insulin drops to lowest levels. Regeneration of immune cells.
Self-renewal and regeneration of hematopoietic or blood cell stem cells.
References:
|
Autophagy Disrupters:
Conditions such as scheduling fasting periods can induce autophagy. There are conditions that can disrupt autophagy thus leading to diseases which result as a failure of autophagy such as Alzheimer's disease and Parkinson's disease.
Neurotoxins can dysregulate a cell's lysosomes, interfering with the fusion with autophagosomes which encapsulate the proteins to be recycled. When the fusion of lysosomes and autophagosomes is impared, the recycling of proteins is impared leading to an accumulation of proteins. In the case of Alzheimer's disease, the recycling of the tau protein is impaired. In the case of Parkinson's disease, the recycling of the alpha-synuclein protein is impaired.
Also see:- How Parkinsonian Toxins Dysregulate the Autophagy Machinery Int J Mol Sci. (Dagda et al, 2013)
- Accumulation of inorganic mercury in lower motoneurons of mice (1992) B. Arvidson
Genetic factors can play a role in the levels of the lysosomal enzyme glucocerebrosidase (GCase), used by lysosomes to break down proteins. Those with the GBA mutation have suppressed levels of GCase and thus reduced abilities of the lysosomes to effectively participate in autophagy.
Also see:- Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease Brain (Murphy et al, 2014)
"The reduced glucocerebrosidase is likely to contribute to lysosomal dysfunction by altering lysosomal contents and membrane properties, exacerbating any age-related diminished lysosomal capacity." - Genetics and Parkinson's disease
Autophagy Counterpoint:
While there is research showing the value of autophagy, research by Tanik et al. (2013) shows that neurons when harboring Lewy bodies, resist autophagy and in fact are more likely to be signaled for death by fasting. Their research shows that autophagy is important to maintain neuron health by processing alpha-synuclein to prevent aggregation, it also shows that once aggregation is achieved, it causes a defect in the process of autophagosome maturation and its availability for lysosomal fusion resulting in a failure of autophagy to properly maintain neuron cell health. The impairment of autophagy not only disrupts aggregate protein clearance but also the clearance of defective organelles such as mitochondria leading to neuron cell death through several different mechanisms. While it is hard to conclude a course of action for Parkinson's patients, it is clear that autophagy research should continue as it may one day play a relevant role in treatment.
Those that may not want to consider intermittent fasting also include:- those with difficult to control diabetes
- adolescent children
- those who are pregnant
- those with liver or renal issues
- those with food disorder issues (eg. binge eaters, anorexia, bulimia) as intermittent fasting may trigger a disorder episode
- those who are frail with a very low body mass index (BMI)
See references:
- Lewy Body-like α-Synuclein Aggregates Resist Degradation and Impair Macroautophagy (2013 Tanik et al.)
"Alpha-Synuclein aggregates are resistant to degradation and impair autophagy by delaying autophagosome maturation." - the bad news
"Alpha-Synuclein aggregates impair overall macroautophagy by reducing autophagosome clearance, which may contribute to the increased cell death that is observed in aggregate-bearing cells."
"a strategy of autophagy activation may not be productive in cells containing α-syn inclusions, and in fact the cell death that is observed in both aggregate-bearing HEK293 cells and neurons is exacerbated upon activation of autophagy with rapamycin or serum starvation"
DOI: 10.1074/jbc.M113.457408 - Parkinson's disease involves autophagy and abnormal distribution of cathepsin L (Li et al. 2010)
"Accumulation of autophagic vacuoles and related marker of autophagy have been found in PD patients"
"These results suggest that autophagy contributes to the death of nigral neurons induced by 6-OHDA."
"it has been proposed that autophagy was able to initiate apoptosis and cell death by switching on self-killing programme of irreversibly injured cells."
"When autophagy was inhibited by 3-MA, about 40-50% of neurons were rescued from death induced by 6-OHDA."
DOI: 10.1016/j.neulet.2010.11.068 - Autophagy gone awry in neurodegenerative diseases (E Wong et al. 2010)
"recent studies have added a note of caution concerning the applicability of macroautophagy upregulation as a generalized treatment. For example, inhibition, rather than stimulation, of macroautophagy increases neuronal survival in some pathological conditions showing high content of neuronal autophagic vacuoles, such as ischemic stroke."
"The distinctive characteristic of the affected neurons is an increase in the number of autophagic vacuoles that does not associate with increased autophagic flux."
"a primary defect in vesicular fusion that is independent of microtubules, involving instead the actin cytoskeleton. Formation of actin bundles at the surface of autophagosomes is required for fusion, but it is only needed for quality-control autophagy and not for starvation-induced autophagy."
DOI: 10.1038/nn.2575
Signaling:
The human body communicates by various methods. The brain commands muscles by sending a cascading signal along the nervous system. The immune system has sentry cells (macrophages) which release inflammasomes which attract killer cells to come and consume pathogens. The body also has glands and cells which can release proteins which represent a message to cells and organs. The body releases AMPK and mTor proteins to deliver the message that nutrition is abundant or scarce and to enter the state of autophagy when nutrition is scarce.
AMPK:
Adenosine 5' monophosphate-activated protein kinase is an enzyme, known more simply as AMPK, that plays a central role in cell energy metabolism with an ability to regulate a wide variety of metabolic processes. It also plays an important role for intermittent fasting and the activation of autophagy for cells under stress. AMPK is found in the brain, liver, fat and muscle tissue and regulates cellular energy usage, improves physical performance, decreases inflammation and supports autophagy and cellular renewal. AMPK production is activated with high intensity exercise and the production of nitric oxide, intermittent fasting, consumption of antioxidants high in polyphenols and certain foods and supplements. Foods and supplements which boost AMPK are fresh vegetables, green tea, berries, resveratrol, quercetin, berberine, ALA, zinc, omega-3, Aspirin, etc. When energy (ATP) levels are low in the cell, AMPK is activated to restore energy to equilibrium by triggering energy-producing metabolic processes such as glycolysis and fatty acid oxidation, while simultaneously inhibiting energy-consuming metabolic pathways such as protein and fatty acid synthesis (ref). AMPK also has an anti-inflammatory effect and promotes the production of antioxidant proteins including NRF2 and dismutase. A similar mechanism is used by the type II diabetes drug Metformin which increases AMPK and activates autophagy (see drugs in trials and the repurposing of type II diabetes drugs to treat Parkinson's). In contrast, a "bad" diet high in sugar and fat will decrease AMPK activity.
See references:- AMPK and Autophagy (2019)
DOI: 10.1007/978-981-15-0602-4_4 - The AMPK signalling pathway coordinates cell growth, autophagy and metabolism (2011)
DOI: 10.1038/ncb2329 - AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550 (2016)
DOI: 10.1128/MCB.00118-16 - AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases (2012)
covers AMPK in the hypothalamus, AMPK activators:- Pharmacological: ETC-1002, AICAR, Metformin, Thiazolidinediones (TZD): eg: rosiglitazone, pioglitazone, aspirin
- Plant-derived: resveratrol, Polyphenols (eg genistein, quercetin, isoginkgetin, and epigallocatechin-3-gallate (EGCG)), alpha-Lipoic acid (ALA)
- Long chain fatty acids: Omega3 EPA, MEDICA-16
The World Anti-Doping Agency (WADA) banned metabolic modulators designed to increase AMPK. This includes AICAR (AICA ribonucleotide) a medication used to tread cardiac ischemic injury. Studies showed that the AMPK activator, AICAR, boosted the athletic performance of mice by increasing glucose uptake and fatty acid oxidation during exercise.
See:
There are vendors who claim to have supplements to boost AMPK and thus induce a greater period of autophagy. Claims include support for normal glucose levels and insulin activity, energy production and a healthy inflammmatory response.
See:
Warning: inhibition of AMPK signaling in the aged brain will cause a concomitant increase in hippocampal neurogenesis. This research shows that for those over 60 years of age, AMPK may have adverse effects. This research makes it very unclear as to the benefits of AMPK evangelized by so many in the nutrition industry. Thus one may want to activate AMPK in your liver, fat, and muscle cells and inhibit it in your hypothalamus. Exercise and calorie restriction may be preferred instigators of autophagy over AMPK activator medications or supplements.
Reference:- AMPK Signaling Regulates the Age-Related Decline of Hippocampal Neurogenesis (Wang et al. 2019)
"results indicated that the increase in AMPK signaling with age is a key factor leading to the age-related decline of hippocampal neurogenesis and thus, increased susceptibility to age-associated neurological diseases."
DOI: 10.14336/AD.2019.0102

AMPK Charge+: AMPK boosting supplement (vendor claim)
mTOR:
Studies have shown that there are a number of signaling pathways involved in the regulation of autophagy, one is AMPK and another is mTOR. The mammalian target of rapamycin (mTOR) is a protein which regulates cell growth, proliferation, protein synthesis and autophagy. Rapamycin can induce autophagy by blocking mTOR which indicates to the body that no nutrients are being consumed. There are a number of rapamycin based drugs in development to promote autophagy or one can pursue the natural approach of time restricted eating.
See references:- mTOR: a pharmacologic target for autophagy regulation (2015)
DOI: 10.1172/JCI73939
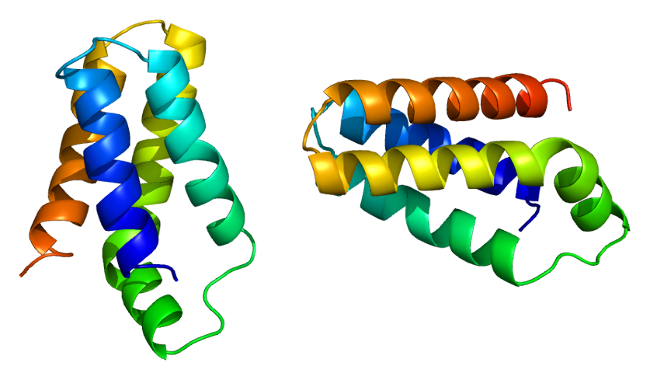
mTOR protein (FRAP1)
This image file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
AMPK and mTOR are the signaling mechanisms used by the body to let it know if there is nutrient availability and signal growth or if there is low nutrient availability and signal the body to stop growing and to invoke the self cleansing pathway of autophagy. Growth mode in fully grown adults leads to diseases such as obesity, type 2 diabetes, cancer, atherosclerosis and neurological ailments such as Parkinson's disease.
Also see Reported effects of autophagy-enhancing agents in preclinical PD models - table of agents which induce autophagy
Autophagy vs the Ubiquitin-Proteasome System:
The cell's two main protein recycling and quality control mechanisms are:- Autophagy: processes long-lived proteins, insoluble protein aggregates and even whole organelles (e.g., mitochondria, peroxisomes) and intracellular parasites (e.g., bacteria)
- The Ubiquitin–Proteasome System (UPS): degrades short-lived proteins and soluble misfolded proteins
Degredation of either mechanism can lead to the failure to process alpha-synuclein proteins effectively and lead to neuron cellular dysfunction which can progress into Parkinson's disease.
UPS processing of short-lived proteins is initiated by adding ubiquitin chains which are subsequently recognized by the cell's organelles called proteasomes which are specialized to degrade the short-lived proteins. UPS and autophagy are interconnected in that the failure or degradation of one sytem often affects the other.
References:- The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function PLOS (Mihaila et al, 2019)
"... marked increases in alpha synuclein expression, mutations in the sequence of alpha synuclein, or even simply high levels of oxidative stress in the local microenvironment, all seem capable of promoting formation of pathological aggregates by interfering with normal turnover of alpha-synuclein via the ubiquitin-proteasome system"
Fasting App:
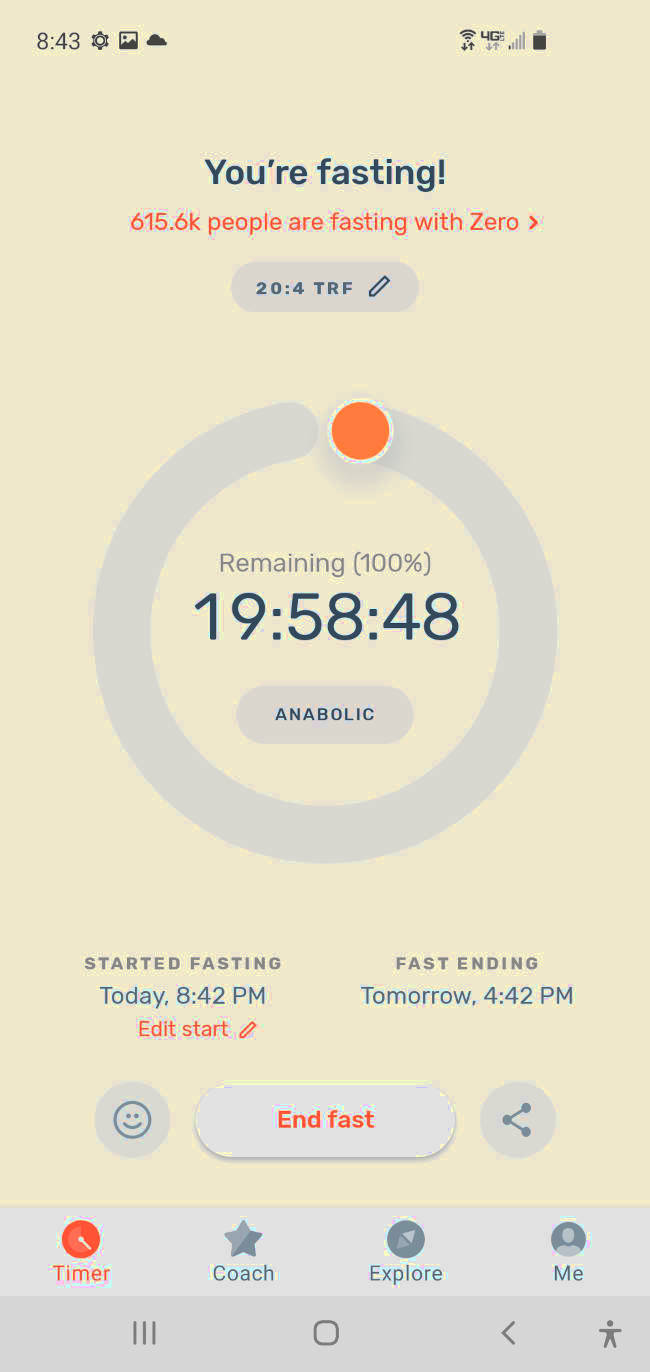
In the modern world of the smart phone, it's not surprising to find out that there is a mobile app to help with intermittent fasting.
The app Zero, named for the amount of food you consume while fasting, will track one's daily fasting.
It also can support the circadian rhythm fast guiding one to eat as close to sunset as possible by using GPS to calculate the phone's location and derive the local sunset time.
This function supports research by Dr. Satchin Panda showing that eating late at night can be detrimental to your health.
Reference: Fasting, circadian rhythms, and time restricted feeding in healthy lifespan Panda 2016
DOI: 10.1016/j.cmet.2016.06.001
See: Zero fasting app
Conclusion:
Healthy People: Restrict one's eating to fast for a 48 hr period once during the week to promote cellular clean-up and counter the generation of Parkinson's pathogens. A supporting daily regiment of "Time Restricted Fasting" schedules eating once a day with a 22 to 23 hour fast.
For those diagnosed with Parkinson's: There is conflicting information. On one hand, those with Parkinson's have neurons with Lewy bodies which may be harmed by fasting. On the other hand, autophagy may provide protection for their neurons which don't have Lewy bodies.
Pros:
- Cellular level restoration, reduced hypertension, anti-inflammatory, circadian rhythm stability and improved sucrose regulation are all potential benefits.
Cons:
- Not likely to make a huge difference if the root cause (eg neurotoxins) which is causing neuron dysfunction and the creation of Lewy bodies and causing Parkinson's disease is not addressed. Extreme fasting will lead to malnourishment and muscle loss (more susceptible as we age). While autophagy may ward off Parkinson's disease by maintaining neuron health, it may not be effective and maybe even harmful to those with Parkinson's and the presence of Lewy bodies.